“My eyes are itchy while I am breastfeeding… but I am worried this eye drop might affect my baby.”
“I was advised to use eye drops for an eye condition. Do I need to stop breastfeeding?”
Many people experience eye discomfort while breastfeeding. You may hesitate to use eye drops out of concern for your baby.
It is generally believed that most eye drops used during breastfeeding are safe for babies when a few key precautions are followed.
This article explains why they are considered safe and provides specific methods to further improve safety, supported by international scientific evidence.
Eye Drops Rarely Reach Breast Milk
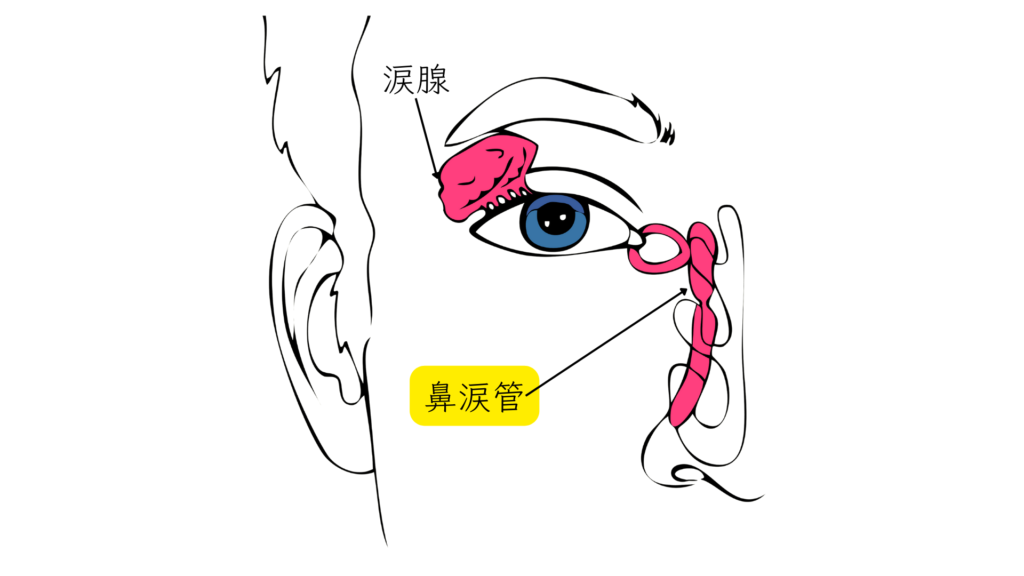
First, it helps to understand the path an eye drop would have to take to reach breast milk.
After instillation, the medication drains from the eye through a duct called the nasolacrimal duct into the nose, where a very small amount is absorbed through the nasal mucosa into the mother’s bloodstream. A portion of that may then pass into breast milk1.
The important point is that the amount reaching breast milk through this process is extremely small compared to the original dose instilled. For many eye drops, less than 5% of the dose is absorbed systemically1.
One international metric is the relative infant dose (RID).
This expresses, as a percentage, the amount of drug an infant receives through breast milk compared with the mother’s dose.
Many experts consider that if the RID is under 10%, the medication can be used safely during breastfeeding2.
Because systemic absorption from eye drops is minimal, the RID for ophthalmic medications is typically well below this safety threshold.
Dramatically Improving Safety: the Nasolacrimal Occlusion Method
There is a simple and effective way to further ensure the safety of eye drops.
It is called nasolacrimal occlusion (Punctal Occlusion).
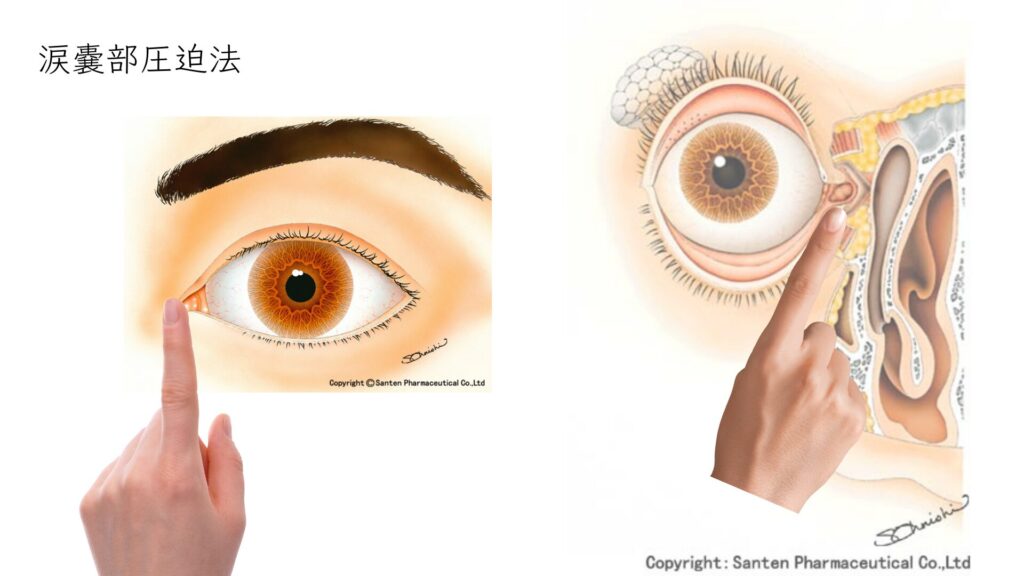
This technique physically prevents the drops from draining into the nasolacrimal duct.
Studies show that gently pressing the inner corner of the eye for 1–5 minutes after instillation can reduce systemic absorption by more than 60%3.
Breastfeeding and medication-use guidelines worldwide strongly recommend practicing nasolacrimal occlusion4.
How to perform nasolacrimal occlusion
- After instillation, gently close your eyelids.
- Without blinking, use your index finger to gently press the small hollow just inside the inner corner of the eye toward the nose for 1–5 minutes.
- Wipe away any excess drops with a clean tissue.
International Safety Assessments by Type of Eye Drop
So, which eye drops can be used safely in practice?
Below, we summarize international evaluations based on global databases such as LactMed (U.S. National Library of Medicine) and the reference book Hale’s Medications & Mothers’ Milk™.
Eye Drops Considered Safe to Use
Artificial Tears and Lubricants
Used to treat Dry Eye Disease and similar conditions. Artificial tears (generic components: potassium chloride/sodium chloride, etc.), which are formulated with tear-like components, and sodium hyaluronate, which helps maintain moisture on the corneal surface, do not contain active drug agents and are considered safe to use while breastfeeding.
Anti-allergy drops (olopatadine, ketotifen, epinastine, antazoline-related agents, etc.)
Olopatadine (Patanol), ketotifen (Zaditen), epinastine (Alesion), and azelastine/antazoline-related products (e.g., Zeppelin brand equivalents)
These medications are commonly prescribed for Hay Fever and other Eye Allergies. Systemic absorption from eye drops is minimal, and transfer into breast milk is low, so the likelihood of affecting the infant is considered low. Using nasolacrimal occlusion concurrently is recommended5.
Steroid Drops (Prednisolone, Fluorometholone, etc.)
Prednisolone (Predonine), fluorometholone (Flumetholon), betamethasone (Rinderon), and dexamethasone (Santezone)
These are used to suppress ocular inflammation.
The term “steroid” may sound concerning, but with short-term ophthalmic use, systemic absorption is very low and transfer into breast milk is minimal.
To date, there have been no reports of adverse effects in infants due to a mother’s use of steroid eye drops6.
Eye Drops Likely Safe when Used under Medical Supervision
Antibacterial drops (levofloxacin, gatifloxacin, moxifloxacin, ofloxacin, etc.)
Levofloxacin (Cravit), gatifloxacin (Gatiflo), moxifloxacin (Vigamox), and ofloxacin (Tarivid)
These antibiotics are used for bacterial Conjunctivitis and similar infections.
According to LactMed, an international database, the risk to infants from ophthalmic use is considered “unlikely.”
Follow your physician’s directions and perform nasolacrimal occlusion for added reassurance4.

Glaucoma medications (prostaglandin analogs: latanoprost, travoprost, tafluprost, bimatoprost, etc.)
Latanoprost (Xalatan), travoprost (Travatan Z), tafluprost (Tapros), and bimatoprost (Lumigan)
These are widely used for Glaucoma. Once in the body, they are rapidly metabolized. Blood levels after instillation are extremely low, so the likelihood of affecting the infant is considered low. International expert sources such as LactMed rate them as “acceptable during breastfeeding”7.

Eye Drops Requiring the Utmost Caution
Glaucoma medications (beta-blockers: timolol, carteolol, betaxolol, etc.)
Timolol (Timoptol), carteolol (Mikelan), and betaxolol (Betoptic)
Timolol is relatively prone to systemic absorption and transfer into breast milk.
There have been reports of infants exposed through breast milk experiencing side effects such as bradycardia and shallow breathing8.
As a rule, these medications should be avoided while breastfeeding.
However, if treatment is essential, use them only in consultation with an ophthalmologist and pediatrician, monitor the infant closely, and take special precautions such as performing nasolacrimal occlusion.
Glaucoma combination eye drops
In glaucoma therapy, combinations that include multiple agents in one bottle are widely used. When selecting a combination product, the key point is whether it contains beta-blockers, which require the greatest caution during breastfeeding.
Combination Drops Containing Beta-Blockers (Use with Particular Caution During Breastfeeding)
Latanoprost/timolol maleate (Xalacom), travoprost/timolol maleate (Duotrav), dorzolamide hydrochloride/timolol maleate (Cosopt), and carteolol hydrochloride/latanoprost (Mikeluna)
Because these contain beta-blockers such as timolol or carteolol, they should generally be used with the same caution as single-agent beta-blockers during breastfeeding.
Combination Drops without Beta-Blockers (Relatively Easier to Use Safely)
Brimonidine tartrate/brinzolamide (Ailamide) and ripasudil hydrochloride hydrate/brimonidine tartrate (Glaalpha)
These combinations use ingredients other than beta-blockers.
You should, of course, consult your physician, but compared with combinations that include beta-blockers, these are generally more feasible to use while breastfeeding.
Why Package Inserts may Differ from International Evidence
Package inserts for eye drops often state “Do not use during breastfeeding” or “If use is unavoidable, discontinue breastfeeding.” These statements are written with particular legal caution and do not necessarily mean the medicine has been proven dangerous for the infant9.
In practice, international databases such as LactMed compile studies and case reports from around the world and assess safety based on scientific evidence. Unlike uniform warnings in package inserts, they provide information aligned with real-world clinical practice10.
Summary
Here are the key takeaways regarding the use of eye drops while breastfeeding.
- Most eye drops can be used safely during breastfeeding.
- To use them safely, perform nasolacrimal occlusion. This method significantly reduces the amount of medication absorbed into the body.
- Among Glaucoma treatments, beta-blockers (timolol) have reported infant side effects and require the utmost caution.
- Do not rely on the package insert alone; make decisions based on international scientific evidence.
Enduring eye discomfort places a burden on your physical and mental health.
Before stopping a medication or hesitating to use it on your own, inform your ophthalmologist that you are breastfeeding and ask which eye drops are safe and how to use them correctly.
References
- Urtti A. Systemic side effects of eye drops: a pharmacokinetic perspective. Prog Brain Res. 2020;257:127-147.
https://pubmed.ncbi.nlm.nih.gov/32943147/ - Hale TW. Hale’s Medications & Mothers’ Milk™. Springer Publishing Company; 2023.
https://www.springerpub.com/hales-medications-and-mothers-milk - Fraunfelder FT. The Importance of Eyelid Closure and Nasolacrimal Occlusion Following the Topical Ocular Administration of Systemic-Acting Medications. Cornea. 2008;27(10):1093-1094.
https://pubmed.ncbi.nlm.nih.gov/19034114/ - Levofloxacin. In: Drugs and Lactation Database (LactMed®). Bethesda (MD): National Institute of Child Health and Human Development; 2006.
https://www.ncbi.nlm.nih.gov/books/NBK501002/ - Olopatadine. In: Drugs and Lactation Database (LactMed®). Bethesda (MD): National Institute of Child Health and Human Development; 2006.
https://www.ncbi.nlm.nih.gov/books/NBK501517/ - Prednisolone. In: Drugs and Lactation Database (LactMed®). Bethesda (MD): National Institute of Child Health and Human Development; 2006.
https://www.ncbi.nlm.nih.gov/books/NBK501076/ - Latanoprost. In: Drugs and Lactation Database (LactMed®). Bethesda (MD): National Institute of Child Health and Human Development; 2006.
https://www.ncbi.nlm.nih.gov/books/NBK501675/ - Lustgarten JS, Podos SM. Topical timolol and the nursing infant. Arch Ophthalmol. 1983;101(9):1381-1382.
https://pubmed.ncbi.nlm.nih.gov/6615302/ - American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108(3):776-789.
https://pubmed.ncbi.nlm.nih.gov/11533352/ - National Institute of Child Health and Human Development. Drugs and Lactation Database (LactMed®).
https://www.ncbi.nlm.nih.gov/books/NBK501922/
![Takeru Eye Clinic | Takatori Shopping Street, Sawara-ku, Fukuoka City [Nishijin Station / Fujisaki Station]](https://takeru-eye.com/wp-content/uploads/2022/10/takeru_logo_for-WP-header.png)





